The Problem:
Photocatalysis of carbon dioxide (CO2) to carbon monoxide (CO) is shown to not be very selective in current studies. Due to the unreactive nature of CO2, the compound requires large amounts of energy to break its chemical bonds, leading to a limited chemical use of CO2 in renewable energy processes. Solar or photoelectric powered catalytic conversion of CO2 to usable fuel precursors (CO) would allow for the storage of solar energy for usage in the dark and for transportation.
The Solution:
Researchers at the University of Alabama have developed a durable, robust transition metal complex scaffold that allows the reduction of CO2 to higher energy products such as CO through the use of either sunlight or electricity. This catalyst makes use of Ruthenium (Ru) and a pyridine ring to increase the selectivity of the photocatalytic reaction. The proposed technology will facilitate the process of breaking CO2 bonds, lowering energy requirements.
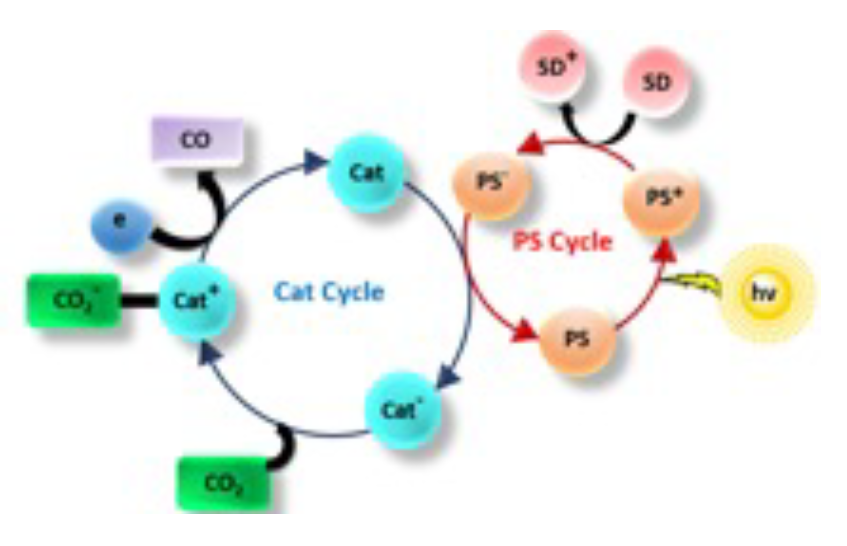 Photocatalytic Reduction of CO2 to CO
Photocatalytic Reduction of CO2 to CO
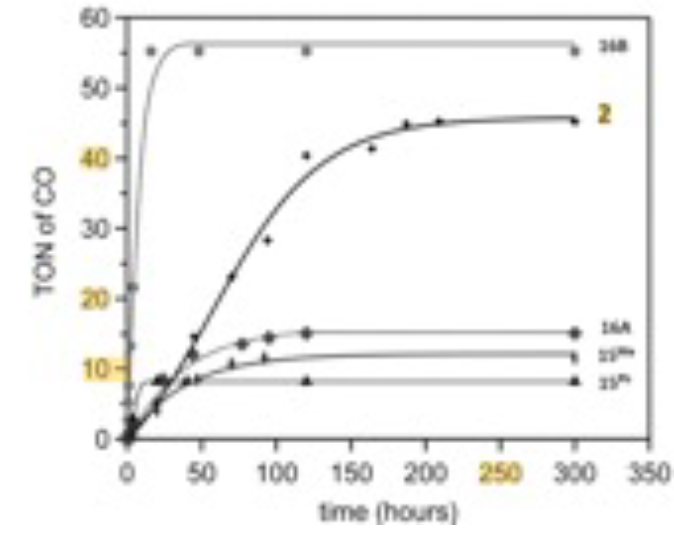 Photocatalytic Production of CO
Photocatalytic Production of CO
Benefits:
• The catalysts are some of the most durable & fastest known.
• The technology is longer lasting & makes a fuel precursor greener than other technologies.
• Increased reaction selectivity.
• Higher turnover rate than current methods.
• Lower power requirement for completion of reaction.
• Conversion of CO2 to solar fuels is carbon neutral, does not contribute to global warming.
The University of Alabama Research Office of Innovation and Commercialization (OIC) is a non-profit corporation that is responsible for commercializing University of Alabama technologies and for supporting University research. At OIC, we seek parties that are interested in learning more about our technologies and commercialization opportunities, and we welcome any inquiries you may have.